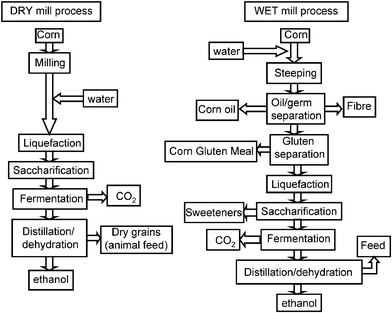
Blog #3 24/2/17
Hello Blogheads!
Unfortunately, this will be my
last entry.
Today I will be assessing the
potential of ethanol to be used as an alternative fuel and discus the pros and
cos of its use.
The use of ethanol encompasses a
number of advantages and disadvantages regarding its use as a complete
alternative to petrochemicals.
Advantages
 The most important advantage of
ethanol as a fuel source is that, unlike petrochemicals, it is a completely
renewable resource, meaning that it will never run out due to our infinite
supply of glucose from plant matter. in addition, no research into the
technology of the extraction process is required, as we already have the
technology required and it has been proven to work. Ethanol unlike
petrochemicals are completely greenhouse friendly. as mentioned in the previous
blog, the carbon dioxide released due to the burning if ethanol is equal to the
amount that was absorbed by the plants during photosynthesis to make sugar,
therefore the plant derived ethanol does not contribute to the growing “Greenhouse
effect” which is detrimentally affecting the Earth. Finally as previously
mentioned, no engine modifications are required when petrol is mixed with up to
20% ethanol, helping conserve petrol overall.
The most important advantage of
ethanol as a fuel source is that, unlike petrochemicals, it is a completely
renewable resource, meaning that it will never run out due to our infinite
supply of glucose from plant matter. in addition, no research into the
technology of the extraction process is required, as we already have the
technology required and it has been proven to work. Ethanol unlike
petrochemicals are completely greenhouse friendly. as mentioned in the previous
blog, the carbon dioxide released due to the burning if ethanol is equal to the
amount that was absorbed by the plants during photosynthesis to make sugar,
therefore the plant derived ethanol does not contribute to the growing “Greenhouse
effect” which is detrimentally affecting the Earth. Finally as previously
mentioned, no engine modifications are required when petrol is mixed with up to
20% ethanol, helping conserve petrol overall.
Disadvantages
 if petrochemicals want to be completely
replaced with ethanol, up to 75% of agricultural land would need to be
dedicated to the ethanol industry which causes major disadvantages to farmers
and commercial agriculturalists. in the 1980’s, Brazil attempted to implement
ethanol as their primary source of fuel, dedicating 275% of their agriculture
to ethanol production. the attempt led to an economic failure, and disrupted a
major segment of their food production farming o make way for “ethanol farming”.
to run vehicles on pure ethanol fuel, a completely new engine is required to be
designed and built to allow for ethanol to function as a fuel. Ethanol tends to
be corrosive so the materials which the engine is constructed out of must suit
this need. Ethanol also has a different energy content, ignition temperature
and burn characteristics compared to petrochemicals. The cost of setting up
factories that will be able to design a new engine design is extremely high,
causing their production to be very unlikely meaning that there is a low chance
that ethanol will be used for more than 10% as anytime soon. a full extender
if petrochemicals want to be completely
replaced with ethanol, up to 75% of agricultural land would need to be
dedicated to the ethanol industry which causes major disadvantages to farmers
and commercial agriculturalists. in the 1980’s, Brazil attempted to implement
ethanol as their primary source of fuel, dedicating 275% of their agriculture
to ethanol production. the attempt led to an economic failure, and disrupted a
major segment of their food production farming o make way for “ethanol farming”.
to run vehicles on pure ethanol fuel, a completely new engine is required to be
designed and built to allow for ethanol to function as a fuel. Ethanol tends to
be corrosive so the materials which the engine is constructed out of must suit
this need. Ethanol also has a different energy content, ignition temperature
and burn characteristics compared to petrochemicals. The cost of setting up
factories that will be able to design a new engine design is extremely high,
causing their production to be very unlikely meaning that there is a low chance
that ethanol will be used for more than 10% as anytime soon. a full extender

Overall, with the current
technologies and knowledge about ethanol, the use of ethanol as a main source
of fuel seems very unlikely UNTIL either, efficient production from cellulose,
derived from crop wastes is researched and used efficiently so that no crops
need to be grown just fir ethanol production OR renewable energy sources such
as solar and wind power operate the distillation process of ethanol.
Thank you for reading my weekly
blog about ethanol Blogheads! it was a pleasure sharing my studies with you.
See you next time!



/editorial/articleLeadwide-doesnt-add-up-e10-fuel-is-cheaper-but-burns-qui37dca.jpg)