Blog#1 10/2/2017
Hello Blogheads! My name is Dr.Shickshak and I will be writing a 3 part blog entry on Ethanol As A Fuel Source. I hope you enjoy!!!
 Modern society is extremely dependent on fuel as a basis of progression and a means of providing energy and electricity to the world. Although resources such as coal and crude oil are providing us with the necessary energy requirements to use as a fuel for automobiles, planes, motorbikes etc. they are non-renewable sources of fuel, drawing attention to the fact that eventually all the coal and crude oil reserves will cease and we will be let looking for an alternative fuel source. A number of Chemists and Chemical engineers such as myself, have been given the responsibility to research and assess the potential of alternative natural, renewable resources as an alternative fuel for society.
Modern society is extremely dependent on fuel as a basis of progression and a means of providing energy and electricity to the world. Although resources such as coal and crude oil are providing us with the necessary energy requirements to use as a fuel for automobiles, planes, motorbikes etc. they are non-renewable sources of fuel, drawing attention to the fact that eventually all the coal and crude oil reserves will cease and we will be let looking for an alternative fuel source. A number of Chemists and Chemical engineers such as myself, have been given the responsibility to research and assess the potential of alternative natural, renewable resources as an alternative fuel for society.
The fuel that is used in societies today is petrol, which is extracted from crude oil which are mined from natural reserves found in the earth. Petrol possesses all the necessary molecular components allowing for it to be a fuel. Although the extraction of petroleum is highly efficient and cost effective, it has been proven in studies collected by the International Chemical Engineers Association, that our natural embankments of crude oil have approximately 100 years of use left, until they are completely drained. Due to this study, a worldwide frenzy over the study of new renewable sources of fuel has occurred, causing me to stumble on perhaps the best renewable fuel resources known to man, Ethanol which is a biopolymer* able to be extracted from sugar cane. Ethanol is a type of Alkanol. Alkanols are a group of alcohols which, due to their structure, are excellent fuels. Their polar covalent bond in the -OH group (shown in the diagram above) creates a charge dipole, and allows for strong hydrogen bonding to exist between each individual alkanol monomer*. One of the major advantages of these properties, is the fact that they cause alkanols to become non-flammable allowing for their use as fuels in industry and everyday life. Ethanol is an extremely important alkanol as it can be created by an Addition reaction between ethylene and water, and be broken down to ethylene, both with the aid of a H2SO4 (Sulphuric Acid) Catalyst. the significance of this reaction is that ethanol can be made from nothing but plant materials! By processes that are renewable and sustainable! the reaction is as follows:
Making Ethylene from ethanol would result in a major chemical resource being available without the use of non-renewable petrochemicals!
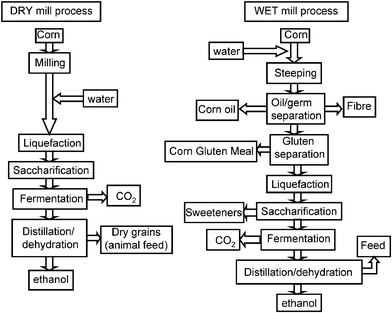
Fermentation is the process used to create ethylene from ethanol, presented in the following flow chart.
Glucose (sugar) is a natural resource found in many plants such as corn, sugarcane etc. contains the essential chemical components to create ethanol through the fermentation process.
C6H1206+ -> 2CH2H5OH (ETHANOL) +2C02
C6H1206+ -> 2CH2H5OH (ETHANOL) +2C02
 Thank you for reading my blog today Blogheads! Tune back in tomorrow for an update on my studies as I will be explaining the use of ethanol as an alternative fuel and the success of its current usage. See you soon
Thank you for reading my blog today Blogheads! Tune back in tomorrow for an update on my studies as I will be explaining the use of ethanol as an alternative fuel and the success of its current usage. See you soon
*biopolymer- renewable polymer made from plants.
*monomer- single molecule of a substance


No comments:
Post a Comment